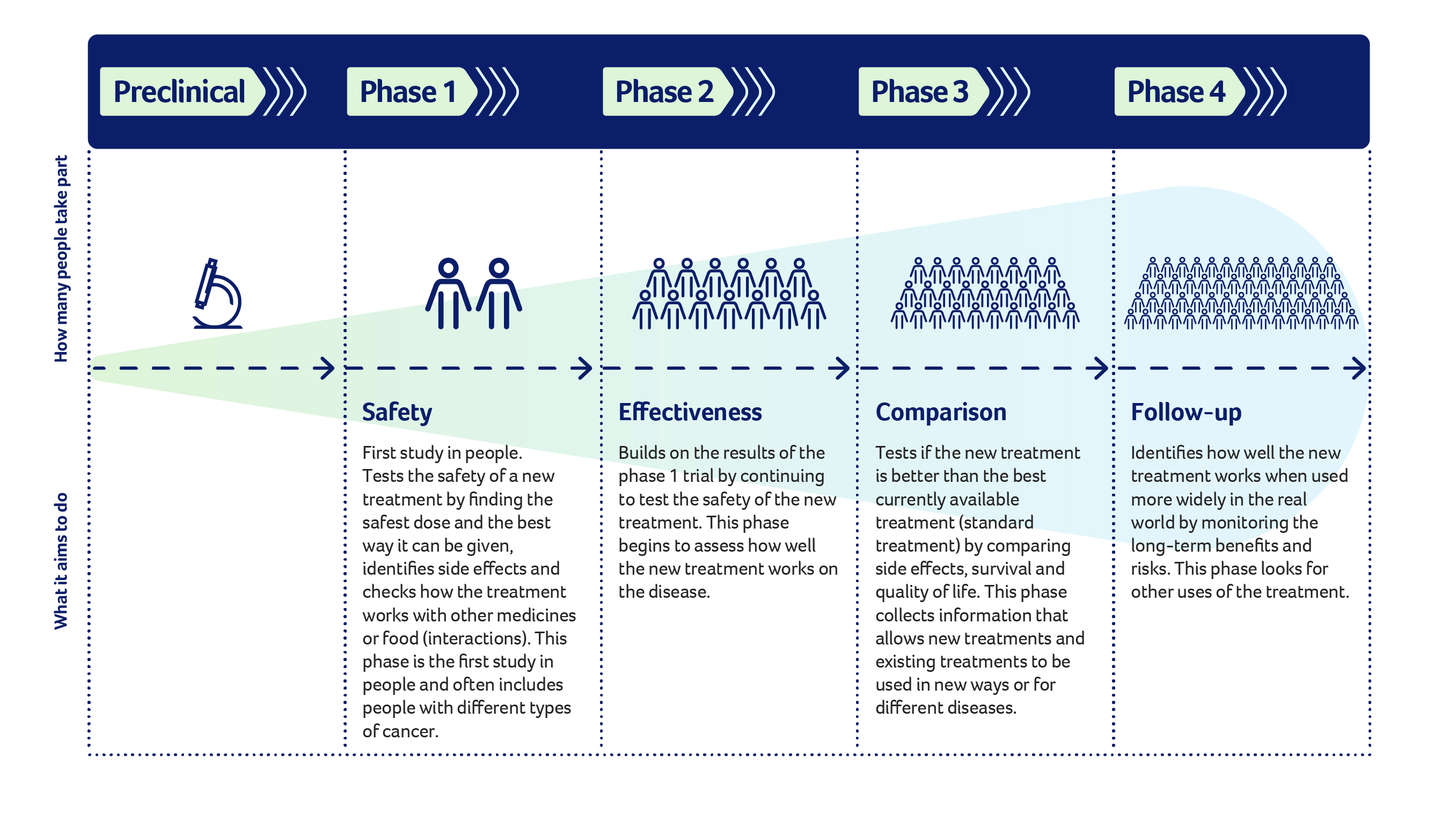
Things to know about phases of clinical trials
All cancer clinical trials follow a stringent, highly regulated process to ensure that the benefits of what is being developed outweigh any risks.
Cancer researchers focus years of time and effort on developing and testing medicines or treatments in laboratories before they progress to the first phase of a clinical trial. The new intervention or treatment is then tested in a series of phases.
The information and data gathered in each trial phase inform whether the study can move to the next phase of research and ultimately whether the intervention can be approved for public use and become standard practice.
Phases of clinical trials